Description
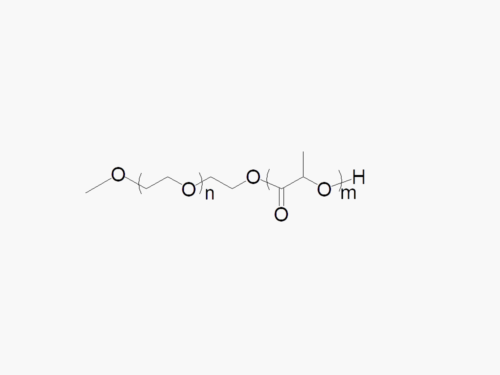
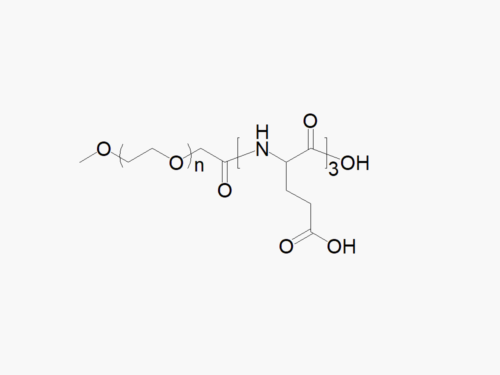
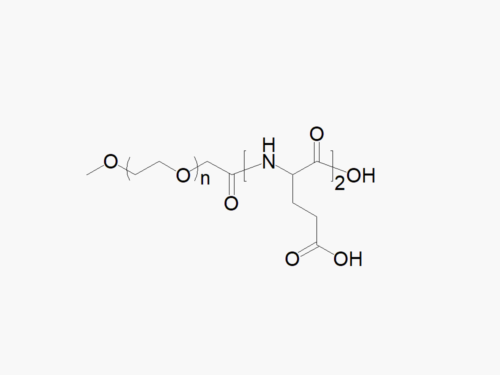
JenKem Technology offers high quality biodegradable PEG co-polymers. The interest in biodegradable polymers for the medical and pharmaceutical field stems from the need for extended release drug delivery systems; and for degradable medical devices that do not require human intervention for removal from the body. JenKem® PEGs are ideal candidates as a polymeric backbone, as they have already been successfully applied for wound sealing and healing, controlled drug delivery, and tissue engineering.
References:
- Gazzola, M., et al., Microtubules self-repair in living cells, Current Biology, 33(1), 2023.
- Poojari, R., et al., Combinatorial cetuximab targeted polymeric nanocomplexes reduce PRC1 level and abrogate growth of metastatic hepatocellular carcinoma in vivo with efficient radionuclide uptake. Nanomedicine: Nanotechnology, Biology and Medicine, 2022.
- Wang, Y., et al., Dual micelles-loaded gelatin nanofibers and their application in lipopolysaccharide-induced periodontal disease, International journal of nanomedicine, 2019, 14:963.
- Richard, M., et al., Active cargo positioning in antiparallel transport networks, bioRxiv, 2019.
- Xiong, Q., et al., Facile fabrication of reduction-responsive supramolecular nanoassemblies for co-delivery of doxorubicin and sorafenib towards hepatoma cells, Frontiers in pharmacology, 2018, 9, p.61.
- Jin, Q., et al., Edaravone-Encapsulated Agonistic Micelles Rescue Ischemic Brain Tissue by Tuning Blood-Brain Barrier Permeability, Theranostics, 2017, 7(4):884.
- Nguyen, F., et al., Enhanced Intratumoral Delivery of SN38 as a Tocopherol Oxyacetate Prodrug Using Nanoparticles in a Neuroblastoma Xenograft Model, Clinical Cancer Research, 2018, p.3811.
- Liang, H., et al., Size-Shifting Micelle Nanoclusters Based on a Cross-Linked and pH-Sensitive Framework for Enhanced Tumor Targeting and Deep Penetration Features. ACS applied materials & interfaces, 2016, 8(16):10136-46.
- Li, Q., et al., Development of reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. Journal of Materials Chemistry B, 2016.
- Schaedel, L., et al., Microtubules self-repair in response to mechanical stress, Nature Mater., 2015, 14(11):1156-63.
- Bhajun, R., et al., A statistically inferred microRNA network identifies breast cancer target miR-940 as an actin cytoskeleton regulator, Scientific Reports, 2015, 5, 8336.
- Portran, D., et al., Micropatterning Microtubules, Methods in Cell Biology, Matthieu P, Manuel T (Editors) Academic Press, 2014, 39-51.
- Boujemaa-Paterski, R., et al., Directed actin assembly and motility, Methods Enzymol, 2014, 540: 283-300.
- Vignaud, T., et al., Polyacrylamide hydrogel micropatterning, Methods Cell Biol, 2014, 120: 93-116.
- Reymann, A.-C., et al, Nucleation geometry governs ordered actin networks structures, Nature Materials, 2010, 9, 827-832.
- Kiss, A., et al., Nuclear Motility in Glioma Cells Reveals a Cell-Line Dependent Role of Various Cytoskeletal Components, PLoS ONE, 2014, 9(4): e93431.
- Portran, D., et al., Quantification of MAP and molecular motor activities on geometrically controlled microtubule networks, Cytoskeleton, 2013, 70: 12–23.
- Schiller, H.B., et al., β1-and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments, Nature cell biology, 2013, 15.6 : 625-636.
- Galland, R., et al., Fabrication of three-dimensional electrical connections by means of directed actin self-organization, Nature materials, 2013, 12.5 : 416-421.
- Portran, D., et al., MAP65/Ase1 promote microtubule flexibility, Molecular biology of the cell, 2013, 24(12):1964-73.
- Pitaval, A., et al., Cell shape and contractility regulate ciliogenesis in cell cycle–arrested cells, JCB, 2010, 191 (2):303-312.