Please contact us at sales@jenkemusa.com to request a quotation.
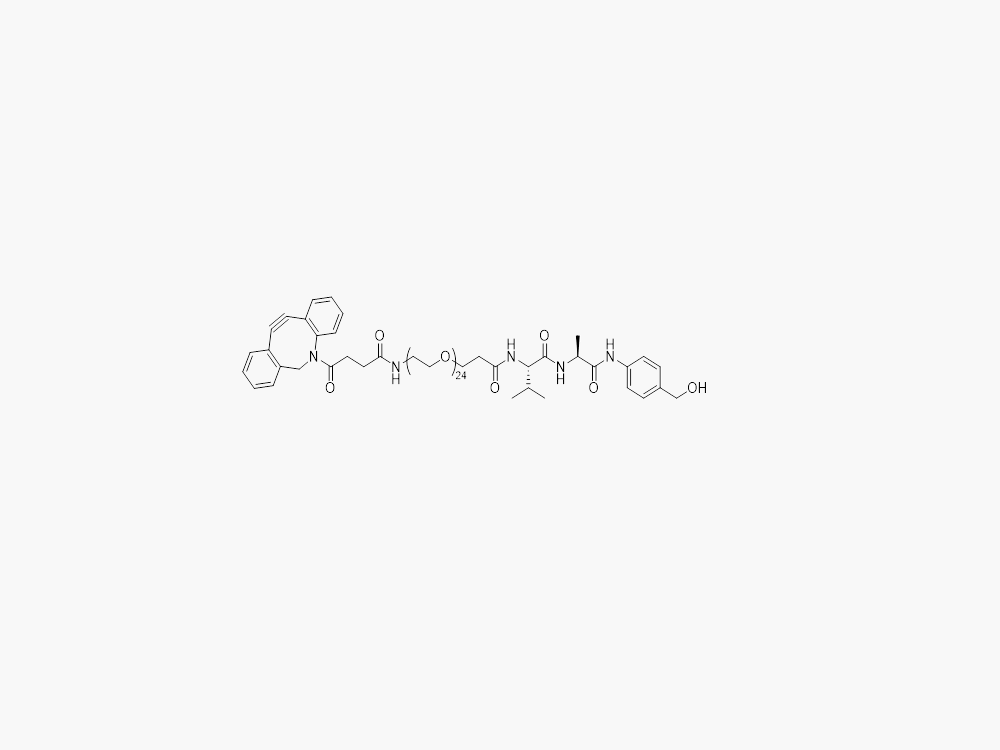
DBCO-PEG24-VA-PAB
Description
JenKem Technology provides large scale manufacture of high purity DBCO-PEG24-VA-PAB (DBCO PEG24 VA-PAB) linker for Antibody Drug Conjugates (ADCs) and other applications, in both GMP and non-GMP grade. Please contact us at sales@jenkemusa.com to request a quotation for high quality DBCO-PEG24-VA-PAB (DBCO PEG24 VA-PAB) ADC linker.
The presence of PEG linkers between the antibody and payload can influence the properties of the ADCs [1]:
- Modulate the physicochemical properties, such as balance the payloads’ hydrophobicity, improve solubility, increase DAR, and reduce aggregation
- Modify the Pharmacodynamic properties, such as increase the rate of payload release and increase invitro cytotoxicity
- Optimize the pharmacokinetic properties, such as clearance, half-life, and biodistribution
Visit https://www.jenkemusa.com/peg-products-for-adcs to learn more about our heterobifunctional monodisperse discrete PEGs, and multi-arm heterofunctional PEGs for ADCs.
References:
1 Giese, M. W., Woodman, R. H., Hermanson, G. T. & Davis, P. D. in Chemical Linkers in Antibody-Drug Conjugates (ADCs) 286-376 (The Royal Society of Chemistry, 2022).
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacture of high-quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified and adheres to ICH Q7 guidelines for GMP manufacture. Production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device, diagnostics, and chemical specialty markets, from laboratory through large commercial scale.