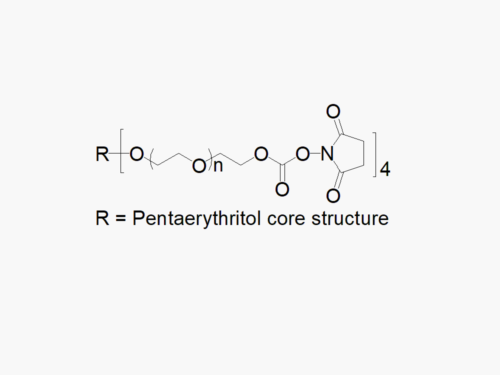
PEG products with additional MW may be made to order, please contact us for details
8arm PEG Vinylsulfone (tripentaerythritol)
$150.00 – $1,200.00
Description
8arm PEG Vinylsulfone (tripentaerythritol) with superior quality specification of > 90% Substitution.
JenKem Technology’s 8arm PEG Vinylsulfone (tripentaerythritol) derivatives can be crosslinked into PEG hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 8 arm (TP) PEGs are synthesized by ethoxylation of tripentaerythritol. 8ARM(TP)-PEGs with tripentaerythritol core have a higher purity as evidenced by MALDI compared with generic 8ARM-PEGs with a hexaglycerin core.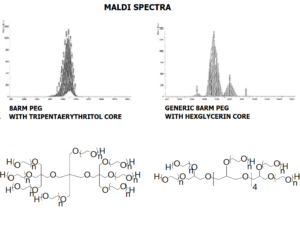
The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm. Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Nason-Tomaszewski, C. E., et al., Extracellular matrix-templating fibrous hydrogels promote ovarian tissue remodeling and oocyte growth, Bioactive Materials, 32, 2024, P. 292-303.
- Gnecco, J.S., et al., Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk, Med, 4(8), 2023.
- Babu, S., et al., How do the Local Physical, Biochemical, and Mechanical Properties of an Injectable Synthetic Anisotropic Hydrogel Affect Oriented Nerve Growth?. Advanced Functional Materials. 2022.
- Below, C.R., et al., A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nature materials, 2022, 21(1).
- Kinney, S.M., et al., Degradable methacrylic acid-based synthetic hydrogel for subcutaneous islet transplantation, Biomaterials, 2022, V. 281.
- Yu, W., et al., Light‐Addressable Nanocomposite Hydrogels Allow Plasmonic Actuation and In Situ Temperature Monitoring in 3D Cell Matrices. Advanced Functional Materials. 2021, 2108234.
- Tomaszewski, C. E., et al., Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation, Acta Biomaterialia, 2021.
- Lau, C. M. L., et al., Local environment-dependent kinetics of ester hydrolysis revealed by direct 1H NMR measurement of degrading hydrogels, Acta Biomaterialia, 2020, V. 101, P. 219-226.
- Tomaszewski, C.E., et al., Adipose-derived stem cell-secreted factors promote early stage follicle development in a biomimetic matrix, Biomaterials science, 2019.
- De Rutte, J.M., et al., Scalable High‐Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds, Advanced Functional Materials, 2019.
- Day, J.R., et al., The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomaterialia, 2018, 67, P. 42-52.
- Manzoli, V., et al., Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol, American Journal of Transplantation, 2018, 18(3):590-603.
- Aguilar, V.M., et al., Microcontact-Printed Hydrogel Microwell Arrays for Clonal Muscle Stem Cell Cultures, InSkeletal Muscle Development, 2017, p. 75-92.
- Cook, C.D., et al., Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function, Integrative Biology, 2017.
- Valdez, J., et al., On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks, Biomaterials, 2017, v. 130, p. 90-103.
- Steele, A.N., et al., A novel protein‐engineered hepatocyte growth factor analog released via a shear‐thinning injectable hydrogel enhances post‐infarction ventricular function, Biotechnology and Bioengineering, 2017.
- Zhou, W., et al., Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation, Macromol. Biosci., 2016.
- Kim, J., et al., Characterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type addition, Soft Matter, 2016.
- Kim, J., et al., Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice, Regenerative Medicine, 2016, 1:16010.
- Rosales, A.M., et al., Photoresponsive Elastic Properties of Azobenzene-Containing Poly(ethylene-glycol)-Based Hydrogels, Biomacromolecules, 2015.
- Allazetta, S., et al., Microfluidic Synthesis of Cell-Type-Specific Artificial Extracellular Matrix Hydrogel, Biomacromolecules, 2013, 14(4), p: 1122-1131.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.