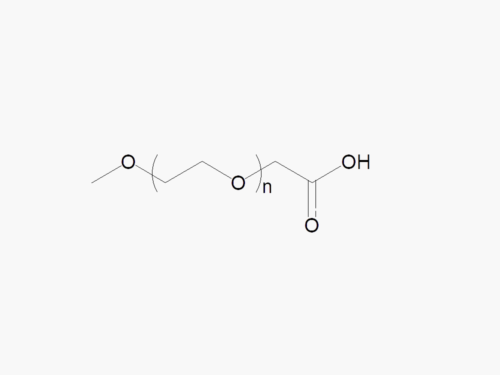
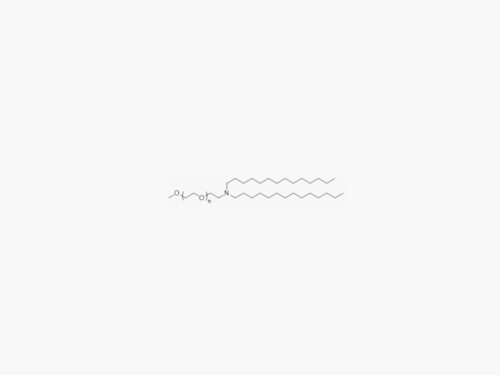
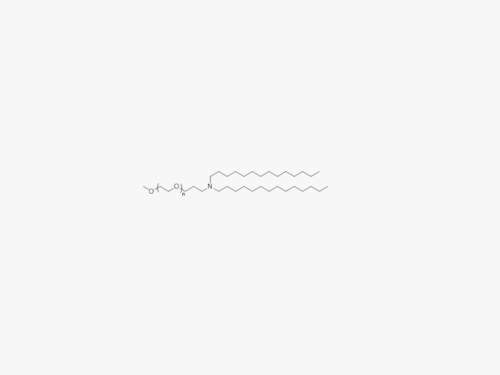
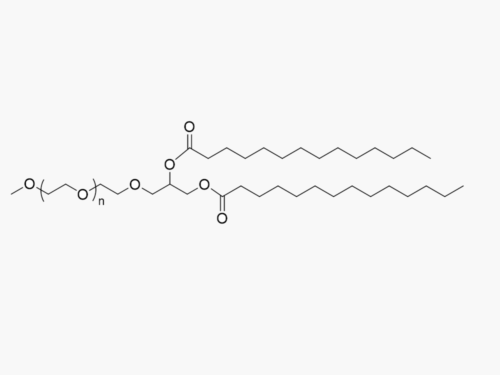
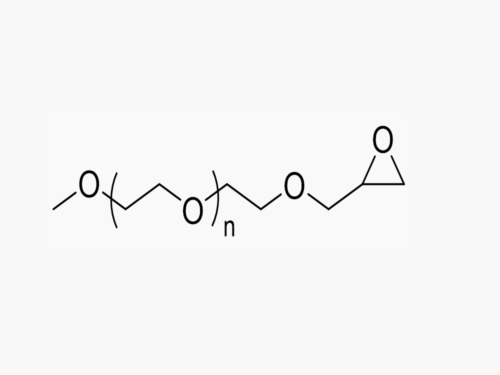
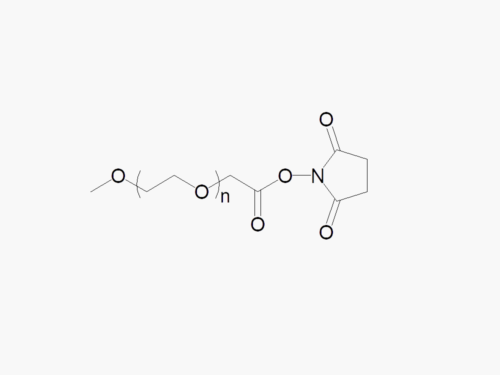
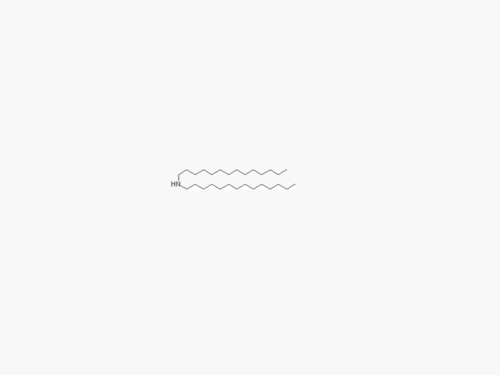
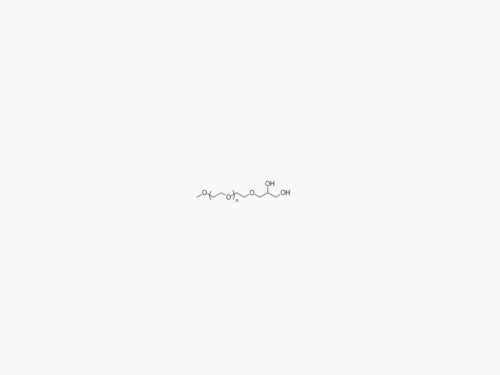PEGs for LNPs
JenKem Technology provides large scale GMP manufacture of PEGs for LNPs, related PEG intermediates, and small molecule reagents for Lipid Nanoparticles.
Lipid Nanoparticles (LNPs) are drug delivery systems commonly employed in the field of nucleic acid drugs. LNPs are generally believed to combine with the cell membrane through non-covalent affinity and absorbed by endocytosis. After being absorbed by the cell, the mRNA delivered by LNPs are released to the cytoplasm and express the target protein, escaping from the endocytosis. The LNP technology has been adopted in recent years for COVID-19 vaccine manufacture.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale